~The team developed 5n, a potential prostate cancer inhibitor, to disrupt cancer’s survival tactics through molecular sabotage
~The molecule disables TLK1, a repair protein essential for halting tumour recovery in prostate cancer cells, paving the way for new intervention strategies
Gandhinagar, Gujarat, May 28, Indian Institute of Technology Gandhinagar( IITGN ) Researchers Develop Smarter Drugs to Overcome Treatment Resistance in Prostate Cancer.
IITGN sources said today, Cancer often outpaces our treatments in a relentless biological arms race, evolving resistance faster than we can respond. Prostate cancer, which affects over 1.4 million men annually, typically begins as a slow-growing and treatable disease. However, it can become deadly once cancer cells develop resistance to therapy. For decades, doctors have relied on androgen deprivation therapy (ADT) to block testosterone, a hormone that fuels the growth of prostate tumours. While ADT effectively starves the cancer of testosterone, tumour cells have found clever ways to bypass this blockade, reactivating their growth despite low hormone levels. This advanced, treatment-resistant form of the disease is known as metastatic castration-resistant prostate cancer (mCRPC), and it is responsible for more than 375,000 deaths worldwide each year.
A research team from IIT Gandhinagar’s Cancer Chemical Biology Lab recently identified a molecule that targets a key survival mechanism used by cancer cells to resist standard therapies. “Typically, cancer cells activate a protein called Tousled-like kinase 1 (TLK1), which acts like a molecular repair crew,” explained Dr Sivapriya Kirubakaran, corresponding author of the study and a Professor at IITGN’s Department of Chemistry. “When therapies like ADT damage cancer cells’ DNA, TLK1 swoops in to fix the breaks, enabling tumours to survive, proliferate, and evolve into mCRPC.”
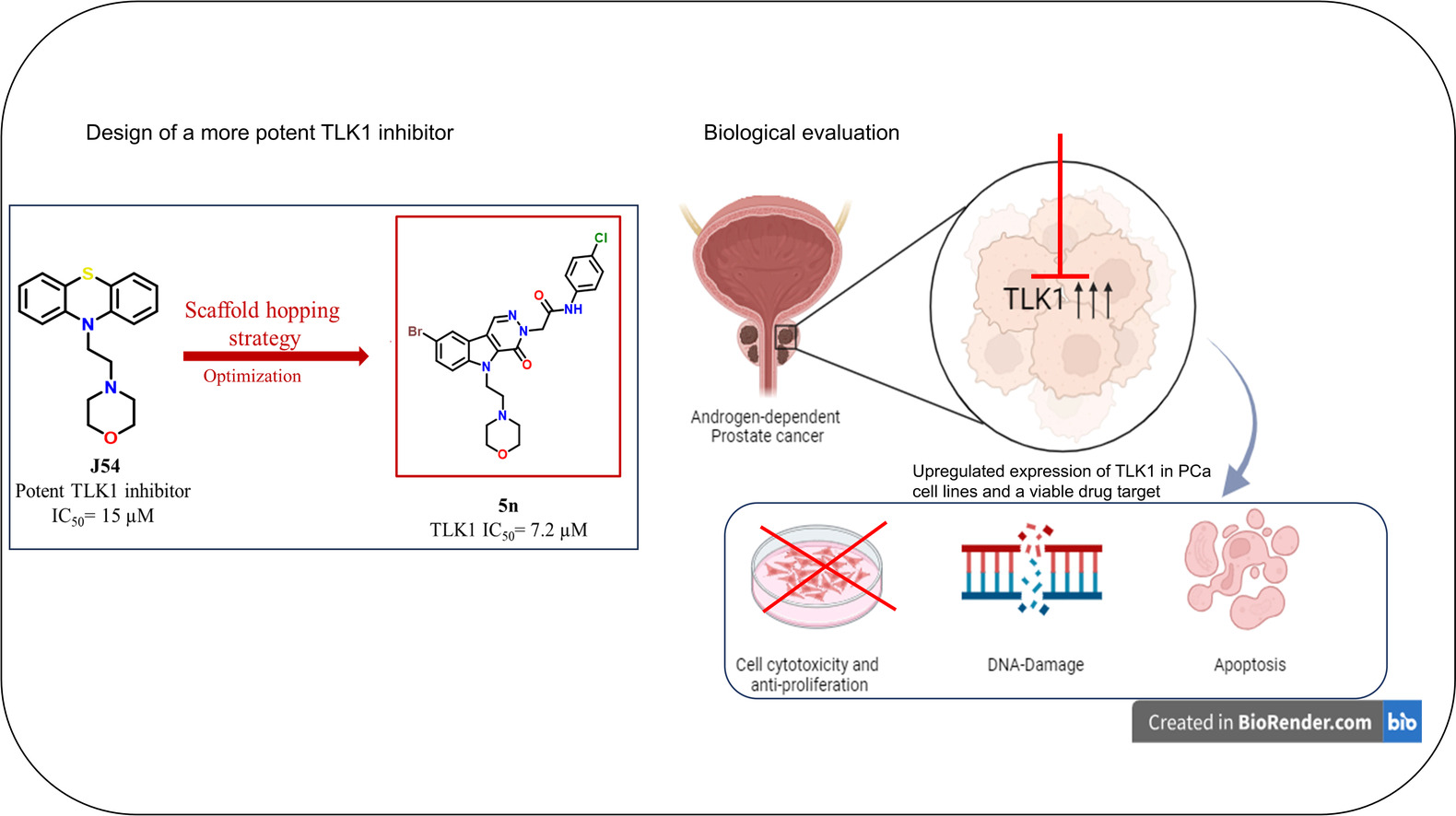
Traditional therapeutics using TLK1 inhibitors have been limited to a small class of compounds known as phenothiazines. Initially developed as antipsychotic drugs, they show limited specificity and potency in prostate cancer therapeutics and are associated with undesirable side effects. The IITGN team sought to address this gap by creating a new class of inhibitors with better safety and effectiveness.
We designed novel drug variants by modifying J54, our in-house designed phenothiazine-based TLK1 inhibitor,” said Dr Delna Johnson, first author of the study and a postdoctoral fellow at IITGN’s Cancer Chemical Biology Lab. J54 was developed by former researchers of the lab and has shown effective action against mCRPC in previous studies. “We replaced J54’s core structure and systematically modified its other parts to create new molecules capable of forming stronger interactions with the TLK1 protein,” she added.
The research team synthesised two series of molecules and performed a comprehensive set of experiments to evaluate their biological activity. In in vitro (test tube) assays, several compounds showed promising inhibition of TLK1, with one molecule, named 5n, standing out as the most potent. Molecule 5n could inhibit TLK1 significantly better than J54, and, crucially, it did so without competing with ATP, the cell’s primary energy molecule. This non-ATP-dependent mechanism would ensure that 5n could block TLK1 activity even when cellular ATP is abundant, making it harder for the tumour to shrug it off. “By retaining J54’s morpholine-based side chain, a common chemical structure in many drugs, and adding an amide linker, our team sculpted a molecular decoy that disables the protein’s function without disturbing the rest of the cellular machinery,” noted Dr Vijay Thiruvenkatam, senior author of the study and an Associate Research Professor at IITGN’s Department of Biological Sciences and Engineering.
To assess whether these molecules would work in living systems, the researchers tested their compounds on the LNCaP cell line, a human prostate cancer cell line that still responds to hormones. The cells, routinely used as a testing model for early-stage prostate cancer, responded strongly to 5n. Even at low doses, the compound reduced the survival of cancer cells and dramatically inhibited their ability to multiply and form new tumours. In combination with Bicalutamide, a commonly used anti-androgen drug, 5n showed a six-fold improvement in reducing cancer cell survival. Additional experiments revealed that this dual treatment triggered significant DNA damage in the cancer cells and activated apoptosis, a self-destruct mechanism reserved for when a cell recognises its own irreparable flaws. Importantly, 5n demonstrated far less toxicity toward healthy, non-cancerous cells, suggesting that it selectively targets cancer cells, which is critical for reducing side effects in potential therapies.
Before any new drug reaches a clinic, it must pass through layers of testing: animal studies, safety trials, and human volunteers. Anticipating these steps, the team conducted in silico ADME (absorption, distribution, metabolism, and excretion) analyses. “We used computational models to predict how their compounds might behave in the human body,” mentioned Ms Shivangi Sharma, a former IITGN Master’s student currently pursuing her PhD at Texas A&M University, USA. Compound 5n passed these tests with favourable properties, including good oral absorption, drug-like properties, and a promising safety profile. Additionally, how 5n engages its target was also explored by using kinetic studies to map the methods by which the molecule alters TLK1’s behaviour in real-time. These insights are blueprints, guiding the design of future versions of the compound with even greater specificity and potency.
With prostate cancer continuing to claim hundreds of thousands of lives each year, the need for more effective therapies is urgent. “By targeting a key protein involved in therapy resistance and designing a new class of molecules with precision and purpose, we have opened a new avenue for treating one of the most challenging forms of cancer,” says Professor Kirubakaran. The future direction of this research will involve further preclinical studies to assess safety and effectiveness in animal models, followed by potential clinical trials.
The study, published in Bioorganic Chemistry, exemplifies how interdisciplinary research can set new benchmarks for cancer therapeutics.